Introduction
MDS is a clonal myeloid disorder manifested by diverse genotypes and phenotypes, characterized by ineffective hematopoiesis. AlloHSCT is the only potential curative therapy for MDS. Myeloablative conditioning regimens are integral part of alloHSCT that allow engraftment and provide a therapeutic effect. Treosulfan has myeloablative and immunosuppressive properties and provides less transplantation related mortality than some of the traditionally used myeloablative conditioning regimens like busulfan-based treatments. In our analysis, we compared the results from the MDS subgroup of the randomized controlled trial (RCT) MC-FludT.14/L (NCT00822393) with real-world data (RWD) of patients treated at the University Hospital Münster and the University Hospital Dresden.
Methods
MDS patients conditioned with treosulfan (30 g/m²) and fludarabine in the RCT (June 2013 to January 2018) and the data obtained from 2 hospitals (RWD) (August 2017 to February 2023) were compared. Baseline characteristics, overall survival, relapse-free survival, relapse (or progression), non-relapse mortality, time to engraftment, and safety profiles between the RCT and RWD were compared. A sensitivity analysis was conducted using propensity score matching (PSM). Additionally, subgroup analyses evaluated the clinical effectiveness of treosulfan by baseline morphologic blast count and the revised International Prognostic Scoring System (IPSS-R).
Results
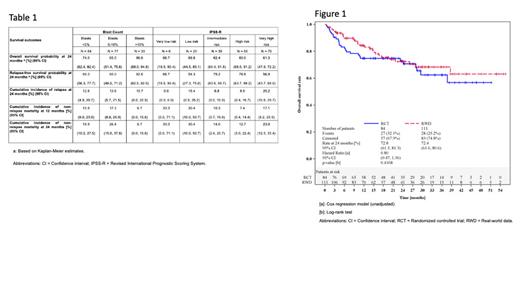
Overall, 195 MDS patients (RCT 84, RWD 111) with at least 12 months follow-up were analyzed. Median age was 62 years (range 39-76 years). Baseline characteristics only differed between the RCT and RWD with respect to age, IPSS-R categories, Hematopoietic Cell Transplantation Co-Morbidity Index total score (HCT-CI), and neutrophil count, indicating some heterogeneity between the RCT and RWD patients. In the PSM analysis, 106 (RCT 53, RWD 53) patients were matched to address the heterogeneity.
The Kaplan-Meier estimates for overall survival (OS) at 2 years were 73% (95% CI 62-81%) and 72% (95% CI 62-81%) for the RCT and RWD (Figure 1), and for relapse-free survival (RFS) at 2 years were 68% (95% CI 57-77%) and 67% (95% CI 56-76%), respectively. Cumulative incidence of relapse at 2 years was 11% (95% CI 4-18%) and 15% (95% CI 7-23%), and for non-relapse mortality (NRM) was 21% (95% CI 12-30%) and 18% (95% CI 10-26%) for the RCT and RWD, respectively. In addition, cumulative incidence of neutrophil engraftment at 28 days was 93% (95% CI 87-98%) and 94% (95% CI 89-98%) for the RCT and RWD, while the median engraftment time was later in the RCT (19 days vs 15 days). Cumulative incidence of platelet engraftment at 28 days was 89% (95% CI 83-96%) and 93% (95% CI 88-98%) for the RCT and RWD, with the same median time 14 days. Further, the PSM sensitivity analysis showed comparable efficacy between the RCT and RWD. Interestingly, bone marrow blast count prior to conditioning and IPSS-R subgroups only had minor, statistically not significant impact on survival endpoints (Table 1).
The number of deaths were 27 (32%) and 28 (25%) in the RCT and RWD, respectively. The most frequent cause of death was transplantation related (20% vs 14%). One patient experienced primary graft failure in the RCT and none in the RWD. More patients had acute GVHD with at least grade 2 in the RCT (27%) than in RWD (18%).
Conclusions
The efficacy and safety of treosulfan-based conditioning therapy prior to alloHSCT in post-authorization real world setting was comparable with the results obtained from the RCT. Our data suggest that bone marrow blast count is no strong predictor for survival after alloHSCT. Therefore, we conclude that the approved treosulfan-based conditioning therapy for patients with MDS, including those with increased blasts, has promising outcomes after alloHSCT.
Disclosures
Stelljes:Novartis: Speakers Bureau; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; MSD: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees; medac: Other: Editorial and statistical support , Speakers Bureau; Gilead: Speakers Bureau; Jazz: Speakers Bureau; abbvie: Speakers Bureau. Sockel:Blueprint: Consultancy, Honoraria; GSK: Consultancy, Honoraria; SOBI: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Active Biotech: Research Funding. Schetelig:Novartis: Honoraria; BMS: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; Eurocept: Honoraria; BeiGene: Consultancy, Honoraria. Lenz:Hexal/Sandoz: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Genase: Consultancy; Immagene: Consultancy; BeiGene: Membership on an entity's Board of Directors or advisory committees; Sobi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PentixPharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Miltenyi: Consultancy, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Constellation: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genmab: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Morphosys: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Lilly: Consultancy; University Hospital Munster: Current Employment; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; NanoString: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Ciceri:ExCellThera: Other: Scientific Advisory Board . Stölzel:medac: Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal